Deep dive: Plant-based meat ingredient optimization
Learn how crop components are processed and functionalized for application in plant-based meat.

Ingredient optimization
Introduction
Processing crops for raw ingredients has focused on oil and carbohydrate extraction. Raw ingredient optimization focused on protein enrichment is essential for plant-based proteins to achieve the scaffolding and functionality observed in animal-derived proteins. Proteins without innate necessary attributes can be enhanced through processing. Processing influences protein yield, fraction composition, physicochemical and structural properties, and functionality. As a result, these optimizations can also make plant-based meat downstream production, such as formulation and texturization, less resource- and time-intensive. This deep dive discusses processing of proteins from crop biomass for use in plant-based meat by increasing protein content and reducing impurities such as antinutrients, bitter flavors, oils, and carbohydrates. It reviews physical, chemical, and biological modifications that improve protein physicochemical and functional properties. For ingredient optimization developments in the plant-based food industry from companies such as Burcon Nutrascience, Roquette, Ingredion, and Avril, see GFI’s 2020 state of the plant-based industry report.
Methods of protein enrichment
Processing differs depending on the starting raw biomass (e.g., leaves, algae, or seeds), source-protein properties, and desired functionalities. Some processes are commonly applied to seeds (Figure 1). Typically, oil is first removed from powdered seeds, leaving the protein fraction in the powder. After enrichment, the powder is categorized as flour (50%–60% protein), concentrate (65%–80%), or isolate (>90%). Highly purified forms like concentrates and isolates are often preferred over whole flours because residual lipids oxidize, sometimes resulting in protein-lipid crosslinking, which lowers the powder dispersibility and changes the protein’s solubility, digestibility, and amino acid content (Swanson 1990). However, protein enrichment can be a resource- and time-intensive process, and protein purity and yield are often inversely proportional (Loveday 2020).
This section discusses techniques for seed-protein enrichment and their tradeoffs. GFI’s contract manufacturing database lists potential partners for large-scale ingredient enrichment.
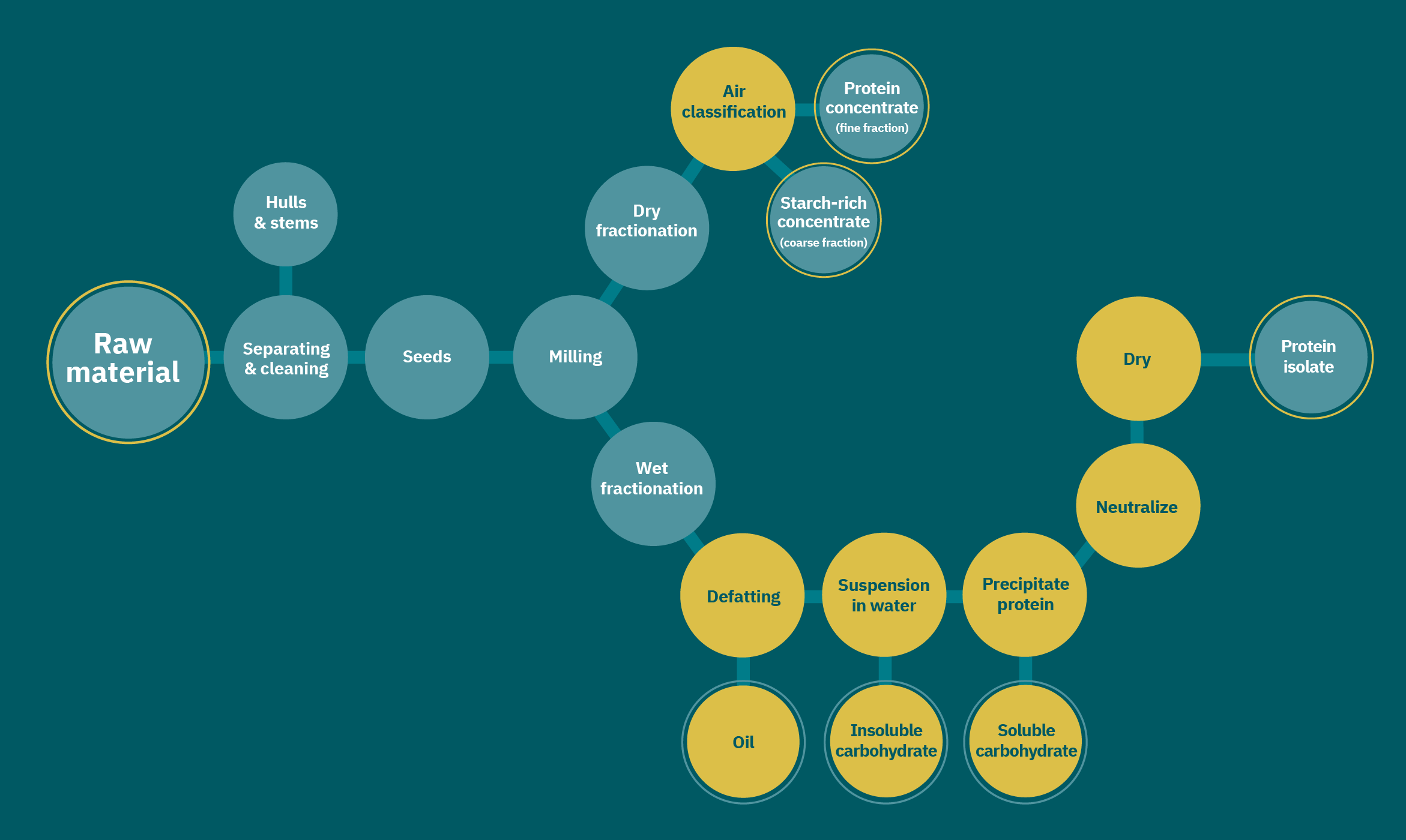
Dehulling and milling seeds
Breaking up the biomass, or “milling,” is typically required to create protein powder from seeds. Protein recovery has been demonstrated to increase as a flour’s particle size decreases (Vishwanathan et al. 2011). Milling’s efficiency for protein enrichment depends on the interactions between proteins and carbohydrates and other cellular components. Accordingly, dehulling and other seed treatments before milling can enhance protein processing.
Physical pretreatments include drying seeds to lower their moisture content. which can make plant cells more brittle and thus lessen the energy required to break cell tissues into smaller particles. However, depending on the application, retention of high moisture content aids processing by reducing cellular interactions. These nuances demonstrate the importance of tailoring extraction techniques to the type of protein. For instance, Pelgrom et al. 2015 compared moisture, defatting, soaking, and freezing-cycle pretreatments to increase protein content of legume fractions. Increasing moisture before dry fractionation improved lupin protein purity, but decreasing it enhanced protein yield. Soaking and freezing cycles negatively impacted protein fractionation.
Biological pretreatments can also improve dehulling and milling. For example, exogenous protease pretreatments improve protein properties by reducing a protein’s size and enhancing its solubility, making extraction easier (Sari et al. 2015). Added carbohydrases degrade plant cell walls, which liberates intracellular components, including proteins. For example, endo-polygalacturonanase, α-galactosidase, and cellulase demonstrated promise for improving the dehulling and splitting of chickpeas before the milling process (Wood et al. 2021). GFI’s solution concept note on biological processing for isolating protein ingredients further discusses the need for greater optimization of biological processing.
Toasting flours to decrease enzyme activity and antinutritional factors has been explored, but this can also negatively hinder functionality (Onimawo & Akpojovwo 2006). Additionally, infrared treatment of mung bean seeds has helped reduce antinutritional factors while retaining fatty acid structures as well as vitamin B1 and B2 content (Padmashree et al. 2016).
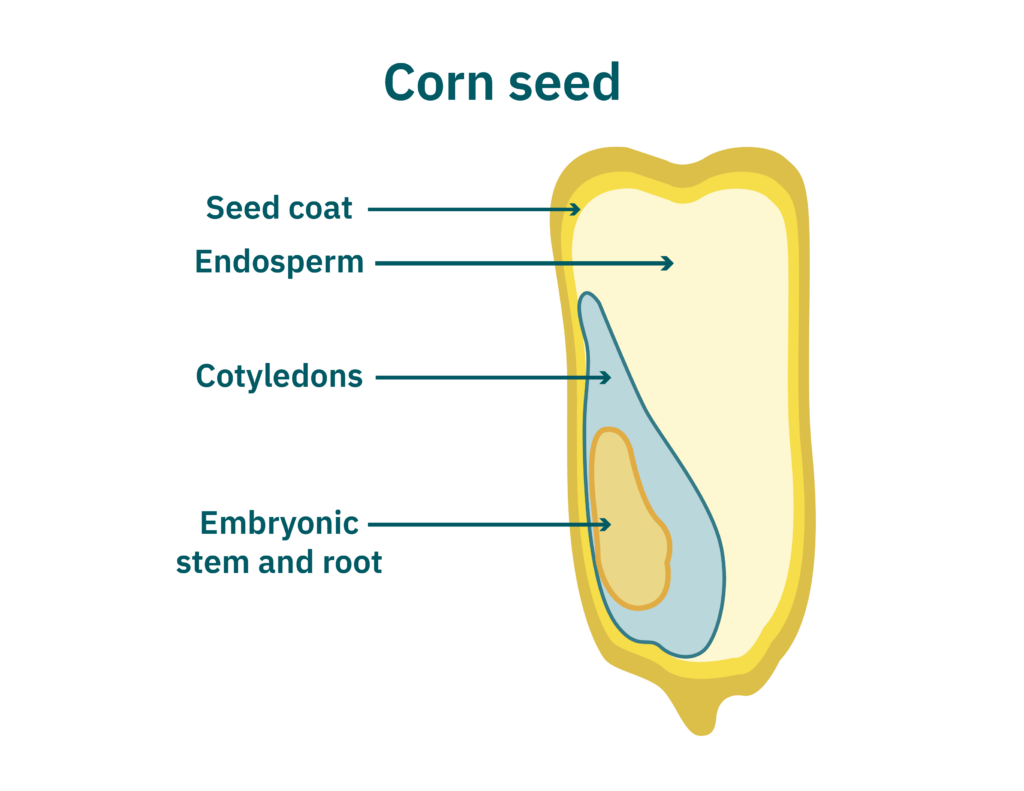
After pretreatments, seeds are milled, wherein the seeds are broken into smaller pieces via grinding, pressing, etc. Selectivity during milling is important for downstream processes. Milling aims to break up seed endosperms and cotyledons (see Figure 2) while keeping starch granules (5-20 μm) and other larger tissues intact for easy separation from smaller protein bodies (1-3 μm). Fine ground flour is essential for dry fractionation (detailed in the next section). Pin milling, jet milling, and abrasive milling or pearling (Moller et al. 2021) achieve small particles for further processing. Cryogenic milling freezes seeds, increasing their brittleness and thus breakability. After milling, major components (oil, protein, carbohydrate, and fiber) are typically separated by wet or dry fractionation.
Wet fractionation
Wet fractionation uses solvents to extract, precipitate, and centrifuge proteins, oils, carbohydrates, and fibers. Protein solubility in aqueous and organic solvents is important to deciding which methods to apply for enrichment. Generally, wet fractionation begins with a flour suspension. Oil-rich flours are first defatted with organic solvents to remove oil. Hexane defatting has increased protein content in safflower, sunflower, canola, and hemp oilseeds (Galves et al. 2019). In this study, oil was collected in the hexane phase and protein in the aqueous phase at pH 3, 5, or 7. Hexane evaporates easily and is distilled from the oil and protein phases. Although hexane is removed, more-benign, sustainable organic solvents are available. A biobased solvent, 2-methyloxolane, has defatted flour rich in soybean oil (Claux et al. 2021). This solvent improved protein concentration without significantly changing the antinutritional factors or amino acid composition compared with hexane.
Consumer perception of organic solvents is often negative, so other options for defatting flours have been explored. Botaneco Inc. has developed a scalable, proprietary aqueous separation process that enriches proteins from a variety of oilseeds. Geerts et al. 2018 also explored aqueous fractionation of soy protein. Here, a soybean flour suspension was created with pH 8-9 water and then centrifuged to separate into three phases: oil, protein-rich liquid, and a pellet rich in water-insoluble fibers. Acidic precipitation separated the protein before it was toasted and texturized to form a structured food sample. Some seeds, especially pulses, have low fat content, so they may not require defatting.
Before alkaline or acidic treatment, defatted flours can be treated for subsequent protein enrichment. For example, various intermediate physical treatments have been tested on defatted soy protein before alkaline extraction, including homogenization, ultrasonication, and steam-cooking (Tao et al. 2019). The effects varied—steam-cooking increased solubility and water-holding capacity, and ultrasonication improved viscosity and emulsion stability.
Optimal enrichment methods are, of course, dependent on protein type. However, proteins are often more soluble at higher pHs, so aqueous washing is most efficient with alkaline solutions. Since proteins typically have a slightly acidic isoelectric point (the pH at which a protein becomes electrically neutral and often precipitates out of solution), precipitation of the aqueous phase is often conducted in acidic aqueous conditions. The optimal pH for washing and precipitation should be tested by confirming the protein’s isoelectric point and then screening multiple wide-ranging pHs for each process (Vilg & Undeland 2017). Solubility of undesired components—fibers, carbohydrates, residual oil—should be considered as well.
Aqueous washing after defatting helps remove small molecule impurities that impart off-flavors, lowered digestibility, undesirable colors, and so on. Parameters for aqueous washing must be tailored to the crop source. For example, aqueous washing of defatted sunflower kernels at various pHs, temperatures, and ethanol concentrations demonstrated that even 0 to 100%) (Jia et al. 2021). These methods removed phenolic compounds where increasing ethanol content limited protein loss, and pH 7 demonstrated better phenolic compound removal compared to pH 4. Even small changes in pH can significantly affect protein yield and enrichment. When comparing pH 8.5, 9.0, and 9.5, notable differences in pea protein recovery, aggregation, and beany off-flavors were observed (Gao et al. 2020). These differences between minute pH adjustments often come with nuanced tradeoffs: For instance, the study demonstrated that pea solubility decreased but yield improved with increasing extraction pH, and the intermediate pH value was best for removing beany off-flavors.
Isoelectric precipitation or ultrafiltration can further enrich proteins. While typically conducted after aqueous washing, precipitation and ultrafiltration can happen at any stage after flour suspension in an aqueous or organic solvent. Using a membrane that separates components based on their molecular weight, ultrafiltration has been compared to isoelectric precipitation in creating lentil protein isolates (Alonso-Mivavalles et al. 2019). Ultrafiltration has demonstrated better functionality retention. A powerful method that integrates acidic washing, alkaline extraction, isoelectric precipitation, and ultracentrifugation can both create canola protein isolates and separate the two main protein fractions, cruciferin and napin (Akbari et al. 2015).
Additional optimizations can be included, such as a freeze-thaw cycle between precipitation and centrifugation to improve protein recovery (Abdollahi et al. 2019).
Many other wet fractionation techniques are available beyond the typical pathways described above. For example, Stone et al. 2015 tested the more traditional alkali extraction/isoelectric precipitation method as well as salt extraction-dialysis and micellar precipitation to create pea protein isolates. Micellar precipitation is a neutral salt extraction method that causes protein isolates to form micelle structures through solution dilution. The researchers found that protein recovery and solubility were highest for salt extraction-dialysis and lowest for micellar precipitation. These methods varied on other traits, including oil- and water-holding capacities, foaming properties, and emulsion capacity. Tanger et al. 2020 tested similar techniques and hypothesized that precipitation and rehydration alter pea protein conformation. Moreover, they observed that albumins were lost during precipitation. Another study demonstrated better hyacinth bean protein yield and content from micellar precipitation than from isoelectric precipitation and salt extraction-dialysis, while isoelectric precipitation gave the highest digestibility (Mohan et al. 2020). These varying results demonstrate the importance of process optimization for different crop sources and intended functional profiles.
Proteins resulting from wet fractionation are usually neutralized in solution and then dried to form a powder. Drying techniques include spray drying, freeze drying, and convective drying. Spray drying has demonstrated effects on proteins’ flavor and functional properties (Carter et al. 2018). Freeze drying has been directly compared to convective drying of chickpea protein concentrates made from alkaline extraction and isoelectric precipitation (Ghribi et al. 2015). The authors demonstrated that freeze drying improves liquid-holding capacities and imparts a lighter color compared with convective drying. Drying method and rehydration at the point of use can negatively impact protein structure and thus end product functionality. Various drying and rehydration methods should be evaluated to reduce downstream complications.
As discussed above, alkaline, acidic, or salt-aqueous conditions and high temperatures are applied during wet fractionation to achieve high protein purity. Additionally, proteins are subjected to drying to remove solvent after fractionation. These conditions can be harsh and negatively influence protein functionality. As a result, dry fractionation is often desired as a milder alternative.
Dry fractionation
Dry fractionation can occur via sieving or sifting, air classification, electrostatic separation, or a combination of these solvent-free techniques. Air classification separates powder particles according to their aerodynamic properties , which are dependent on particle density, particle size, and powder dispersibility (i.e., how uniformly the powder distributes in solution) (Schutyser et al. 2015; Sosulski & Youngs 1979). For example, starch granules are typically larger and therefore less aerodynamic than protein bodies.
GFI grantee Miek Schlangen applies air classification to form mung bean protein concentrate. Electrostatic separation uses a low-energy charged beam to separate powder particles by size. Dry triboelectrification separation electrically charges neutral species by passing them through charged materials and then applying a strong electric field to separate oppositely charged particles. Triboelectrification has been used to create navy bean protein concentrates and demonstrates better solubility, emulsification, and foaming than wet-fractionated concentrates (Tabtabaei et al. 2019). Air classification and electrostatic separation can be combined to further enrich proteins (Pelgrom et al. 2015). Oil can negatively affect dry fractionation by lowering dispersibility. Moreover, dry fractionation usually produces lower-purity protein concentrates compared with wet fractionation. Consequently, many upstream opportunities to improve dry fractionation—plant breeding, pretreatments, or improvements to the dry fractionation process—are available and should be explored.
Comparing wet and dry fractionation methods
While high purity is not always essential for plant-based meat applications, efficient removal of some macromolecular components, antinutritional factors, pigments, and volatile components is often desired (Schutyser et al. 2011). Otherwise, some of these residual compounds could negatively affect the protein’s storage, functionality, and digestibility. Dry fractionation also requires that flour be more finely ground than flour for wet fractionation. Because air classification relies heavily on particle size, it is not suitable for crops with high starch concentrations or small starch granules that are too close in size to protein bodies, such as cowpeas (Cloutt et al. 1987).
But dry fractionation offers many advantages. It uses fewer resources, including water, than wet processes to enrich proteins. Decreased reliance on solvents makes dry fractionation more conducive to creating clean label products (Schutyser et al. 2015). It is more energy-efficient, as wet fractionation requires a high amount of energy to remove water and other solvents. As noted, because of the potentially harsh wet conditions, drying, and rehydration associated with wet fractionation, proteins typically retain better functionality through dry fractionation. For instance, faba bean protein that underwent air classification had higher solubility at neutral pH, as well as improved foaming and gelling properties, but lower protein content and in vitro digestibility compared with the same protein concentrate enriched via acid extraction or isoelectric precipitation (Vogelsang-O’Dwyer et al. 2020).
Wet and dry fractionation can be combined to overcome challenges. For example, air-classified pea protein subsequently subjected to aqueous separation provided a concentrated yet highly functional protein powder (Pelgrom et al. 2015).
Other enrichment methods
Other enrichment processes have been explored, especially for aquatic biomass, which does not lend itself to methods for terrestrial seed crops. For example, pulsed electric fields combined with mechanical pressing and then dialysis and freeze drying have extracted protein concentrations from green marine macroalgae (Robin et al. 2018). Enzymes and ultrasonication have extracted proteins from red, brown, and green seaweeds (Rodrigues et al. 2015). Enzymatic cell disruption has extracted lipids and proteins from microalgae (Sierra et al. 2017). Algae cell walls are rigid and contain strongly-binding anionic alginate polymers, making them particularly difficult to disrupt to access the proteins (Field et al. 2017). (See GFI India’s microalgae and seaweed proteins report.)
Sidestream valorization
Through enrichment of any crop component, sidestreams are bound to arise, as are opportunities to extract value from these streams to complement the profitability of the primary fraction. When oil or carbohydrates are extracted from a plant, the remaining biomass is generally rich in proteins, fiber, carbohydrates, lipids, and vitamins. But it’s typically burned, dumped in landfills, or sold as animal feed. Leveraging such residue in higher-value industries offers potential financial and environmental benefits, including valorizing crops for multiple applications can provide growers with more revenue streams. Moreover, in developed countries, approximately 39% of food waste arises during food manufacturing (Mirabella et al. 2014), so upcycling “waste” ingredients can improve food system sustainability, guiding us toward zero-waste economies. GFI grantee Dr. Marieke Bruins valorizes proteins from agricultural sidestreams to reduce the cost of plant-based meat and increase protein availability.
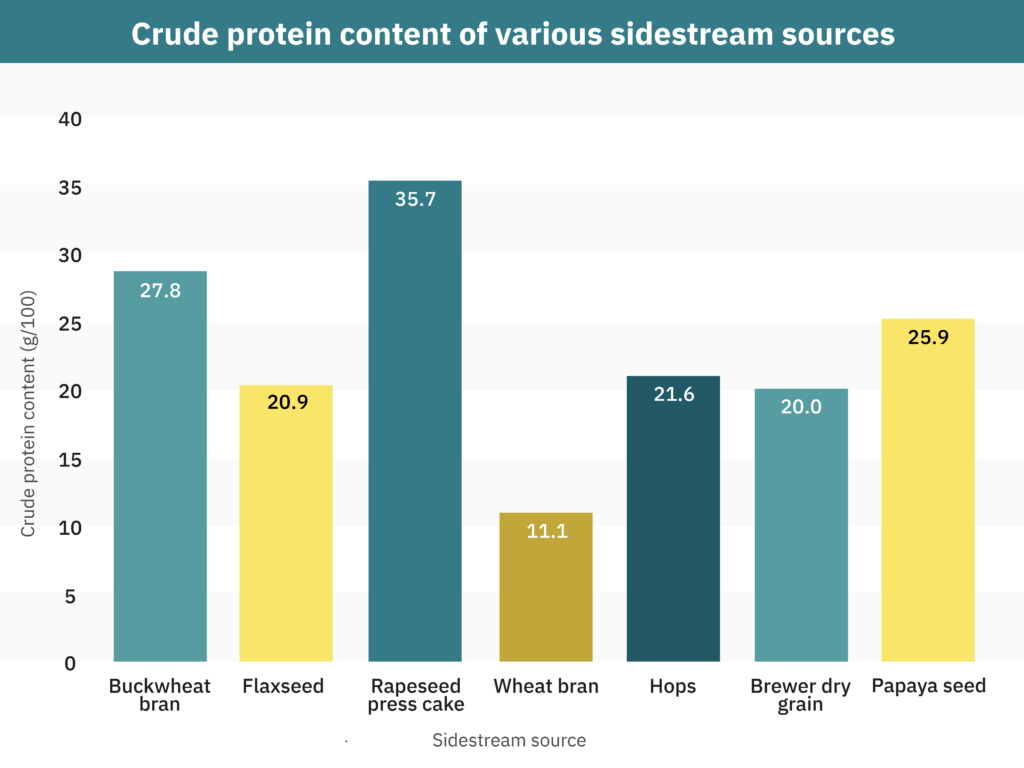
Protein-rich sidestreams from sources processed on a large scale, such as soybean meal, corn gluten meal, or brewers’ spent grains, could be transformed into ingredients for plant-based meat. Biomass sidestreams that could be used for plant-based meat include sidestreams mentioned in Table 1 and those from oil and starch from peanuts (Zhang et al. 2019) as well as lupins and yellow peas (Jonkman et al. 2020); agricultural residues, such as cassava leaves as explored by GFI grantee Dr. Ana Carla Kawazoe Sato and sugarbeet leaves (Martin et al. 2018); and spent distiller’s (brewer’s) grain (Xiros et al. 2012; He et al. 2021). He et al. 2021 conducted a techno-economic analysis of wet-fractionated protein from brewers’ spent grain that revealed that protein processing requires optimization to lower the minimum selling price—hence the need to find and optimize protein-rich sidestreams from processes with large production volumes.
Beyond the potential to source proteins from existing sidestreams, there will increasingly be opportunities to create value from protein-extraction sidestreams. AGT Foods, the world’s largest pulse processor, has developed Veggipasta made from the starch sidestream of pea protein production, generating more value from the starch fraction—previously sold as animal feed. Sidestreams rich in lignocellulose or starches can become valuable feedstocks for fermentation platforms (Ahlborn et al. 2019; Filho et al. 2018) or even for cultivated meat media inputs. Dr. William Chen from Nanyang Technological University applies valorization of soya waste for cultivated meat feedstocks.
Several companies ranging from breeders and processors to end-product equipment manufacturers are exploring opportunities to improve the use of all crop fractions through processing innovations. PURIS—a Minnesota-based breeder and processor of non-GMO crops, such as soy, yellow pea, lupin, corn, and canola—aims to fully valorize whole seeds for their proteins, starches, sugars, fibers, and more. Buhler is focused on upcycling protein sources from oilseed, pulse, and microalgae production sidestreams. Collaborations across the value chain are necessary to optimize sidestream use, increase crop value, and encourage zero-waste economies.
Protein treatments and modifications
After or in conjunction with a protein extraction, opportunities to impart additional functionality are still open. Protein modifications are changes to the sequence or structure achieved through chemical, physical, or biological processing. These treatments can optimize and diversify their structural and physicochemical properties, making proteins more water-soluble and improving their techno-functional properties, such as emulsification, water- and fat-holding, gelation, and foaming abilities. The resulting proteins can be easier to apply in formulations and can impart enhanced end product digestibility and organoleptic properties. The effects of these treatments are also dependent on the purity of the protein fraction—for example, excess carbohydrate presence could increase the gelation abilities of the treated product. Nasrabadi et al. 2021 and Akharume et al. 2021 recently reviewed these methods in detail. This section summarizes some notable examples and builds on information in those reviews (see Table 2 for an overview of relevant studies).
| Treatment | Scalability* | Cost* | Modified characteristics | Select references |
|---|---|---|---|---|
| Chemical treatment | ||||
| pH | +++ | $ | Gelation, water-holding capacity | Li et al. 2020 |
| Ionic strength | +++ | $ | Viscosity, emulsification | Ji et al. 2021 |
| precipitation | +++ | $ | Decolorization, gelation | Du et al. 2020 |
Physical treatment | ||||
| Conventional heat | ++ | $$ | Aggregation, cross-linking, antinutrient content, emulsification, creaming | Peng et al. 2016; Ma et al. 2011; Drozlowska et al. 2020 |
| High pressure | +++ | $$ | Gelation, water-holding capacity, solubility, emulsification | Saricaoglu 2020; Peyrano et al. 2016 |
| Sonication | +++ | $ | Digestibility, solubility, oil absorption, emulsification, gelling | Flores-Jimenez et al. 2019 |
| Cold atmospheric plasma | ++ | $ | Structural changes, emulsification, stability | Mehr et al. 2020 |
Chemical conjugation | ||||
| Glycation | ++ | $$$ | Stability, emulsification, solubility | Zha et al. 2019; Kutzli et al. 2020a; Kutzli et al. 2020b |
| Acylation | + | $$$ | Structural changes, solubility, emulsification, water-holding capacity | Shah et al. 2019; Zhao et al. 2017; Wang et al. 2018; Purkayastha et al. 2016 |
| Phosphorylation | + | $$$ | Water-holding capacity, dispersibility | Hadidi et al. 2021 |
Biological treatment | ||||
| Enzymatic | ++(+) | $($) | Water solubility, yield, emulsification, changes to structure and size, crosslinking, gelation, dispersibility | Akbari et al. 2019; Ghribi et al. 2015; Nieto-Nieto et al. 2014; Zhang et al. 2021; Zhang et al. 2018; Fang et al. 2020; Kaleda et al. 2020 |
| Fermentation | +++ | $ | Antinutrient content, gelation, water-holding capacity, aroma and flavor profile | Xing et al. 2020; Kaczmarska et al. 2018 |
Table 2. Overview and select examples of plant protein modifications through chemical treatment, physical treatment, chemical conjugation, or biological treatment
Chemical treatments
After enrichment, proteins can be subjected to more of the conditions described in the above section—like salt washing, chemical hydrolysis in acid and alkaline conditions, and precipitation—for further modification. These conditions can be combined with the treatment methods discussed below. For example, soy protein isolate has been heated (80 and 95°C) and subjected to sodium chloride solution (0-320 mM NaCl) (Ji et al. 2021). Increasing salt concentration resulted in thicker, more entangled soy protein isolate fibrils and thus enhanced solution viscosity and emulsifying properties. Similarly, peanut protein isolate has been subjected to various pH conditions (pH 2, 4, 10, and 12) for one hour before adjusting back to neutral pH (Li et al. 2020). The researchers found that pH 10 optimally improved the isolate’s gel strength and water-holding capacity due to decreased particle size, increased solubility, and the presence of free thiol groups. Conversely, pH 2 and 12 treatments resulted in protein aggregates without gelation ability. Precipitation with ethanol at low temperatures has decolorized commercially available zein protein powder from yellow to white (Du et al. 2020). Antisolvent precipitation has also been tested on zein protein, where only a small amount of ethanol was necessary to deposit solid protein (Mattice et al. 2020). This precipitation method yielded a highly organized, porous zein gel network. With glacial acetic acid instead of ethanol, zein proteins were protonated and highly unfolded, resulting in a plasticized gel that formed a continuous network. Physical and biological processes that do not require the addition of exogenous chemicals have been explored as more economical and sustainable alternatives.
Physical treatments
Physical treatments are processes that are not chemical or biological, such as heat treatment, irradiation, and pressure application. Because chemical and biological treatments can leave residues and require more inputs, physical treatments have gained attention as potentially lower-cost alternatives. However, physical treatments are not as well controlled as chemical treatments, resulting in wide variations in their effects on different proteins.
Heat treatment is commonly applied to modify protein structure and reduce antinutrient and volatile component content. Proteins often unfold in the presence of heat, which can enhance protein flexibility and functionality. Because globular proteins are packed tightly in a spherical shape, applying heat can induce a molten globule state, which exposes internal amino acids while releasing sequestered antinutrients and therefore changes protein surface hydrophobicity, charge, and reactive groups.
As discussed in the crop development deep dive, physicochemical interactions determine proteins’ secondary, tertiary, and quaternary structures. For instance, hydrophobic amino acids could be uncovered, thus decreasing protein solubility in water. Similarly, more reactive sulfur-containing amino acids could be revealed, thus increasing protein disulfide interactions. As a result, excessive heat can also cause protein aggregation, which can affect functionality.
Optimization of heat treatment is crucial and dependent on the desired product. Pea protein thermal application at 95°C for 30 minutes increased protein aggregation and disulfide bonding, but this improved emulsification and creaming properties (Peng et al. 2016). Roasting green lentil, red lentil, chickpea, and yellow pea protein flours (20-27% protein content) at 80°C or boiling them at 90°C inactivated trypsin inhibitor (an antinutrient) but also decreased protein solubility (Ma et al. 2011). Thermal hydrolysis, applied with high temperature and pressure, of potato protein isolate improved emulsification properties due to protein denaturation (Drozlowska et al. 2020). Ohmic heating has influenced soy protein functionality (Li et al. 2017) but is underexplored for plant protein modification.
Clearly, there is no one-size-fits-all approach to heat treatments , and more standardized empirical studies are necessary to elucidate broader trends.
Thermal treatments other than conductive or ohmic heating are available, such as those using electric fields and electromagnetic radiation (Han et al. 2018). Radio frequency heat treatments have been tested on soy protein isolate (Guo et al. 2017). This treatment resulted in increased surface thiol groups and hydrophobicity without modification to the amino acid sequence. Microwave heating has boosted soy protein gelation strength (Mu et al. 2020), reduced total amino acids and in vitro digestibility of gluten (Xiang et al. 2020), and increased in vitro digestibility of pigeon-pea protein flour (Sun et al. 2020). Both treatments have faster heating rates than conventional heating processes because they penetrate the material more uniformly. Other nonthermal treatments that could be applied to plant proteins include pulsed-electric field, infrared irradiation, ultraviolet irradiation, gamma irradiation, and electron beam irradiation. While radio frequency and microwave treatment modified only secondary and tertiary protein structure, irradiation can also alter the primary amino acid sequence. Changes in protein primary structures can lead to nutritional and allergenic changes that should be carefully evaluated before consumption to ensure food safety. Han et al. 2018 and Nasrabadi et al. 2021 include informative discussions on the mechanisms of protein structure changes for these types of transformations.
Cold atmospheric plasma treatment has also been explored as a physical treatment for plant proteins (Tolouie et al. 2018; Bu et al. 2022). Grass pea protein isolate modified with cold atmospheric plasma had more orderly secondary structures, more globulin dissociation, better emulsification properties, and improved thermodynamic stability (Mehr et al. 2020). The protein isolate’s surface charge and reactive groups increased with higher voltage and longer durations, resulting in superior protein functionality and stability.
Sonication, or the application of high-frequency sound waves (>16 Hz), has also been tested on proteins, including those from black bean (Li et al. 2019), millet (Nazari et al. 2018), potato (Hussain et al. 2021), and glycinin (Liu et al. 2020). Flores-Jimenez et al. 2019 tested high-intensity ultrasound on canola protein isolates and saw no significant changes in protein digestibility but improved solubility, oil adsorption, emulsification, gelling, and foaming properties. Cold atmospheric plasma treatment and sonication are both relatively scalable and cost-efficient.
Mechanical treatments also influence structure and functionality. Planetary ball milling of wheat gluten increased the number of free thiol groups and protein hydrophobicity, as well as improved emulsifying and foaming properties and reduced particle size (Liu et al. 2021). High-pressure homogenization (100+ MPa) similarly decreased protein particle size, unfolded the proteins, and improved solubility, as well as emulsifying and foaming, of lentil protein suspensions (Saricaoglu 2020) and hazelnut protein meal (Saricaoglu et al. 2018). High-pressure homogenization outperformed thermal treatment by further enhancing the gelation and water-holding capacities of cowpea protein isolate (Peyrano et al. 2016). GFI grantee CSIRO is currently exploring high-pressure processing and high-pressure thermal processing parameters for plant proteins as a cleaner-label approach to plant-based meat production. Microfluidization (i.e., using fluid pressure, shear force, impact, and cavitation to reduce a powder’s particle size) ihas also been tested, demonstrating improved fenugreek seed protein color, oil-binding capacity, emulsion stability, and viscoelasticity (Ghanghas et al. 2021). Microfluidization, homogenization, and ultrasonication were compared for pea protein isolate modification (McCarthy et al. 2016), demonstrating that pea protein-stabilized emulsions made using homogenization or microfluidization had better cold-set gelation properties, while sonication improved protein solubility and emulsifying stability.
Physical treatments have a wide variety of effects on protein sequence, structure, and function depending on a multitude of factors, including protein type and process parameters. As a result, more coordinated research with standardized conditions across many protein types is necessary to enable researchers to identify larger trends and draw broader conclusions about the effects of heat, irradiation, and pressure treatments.
Chemical conjugation
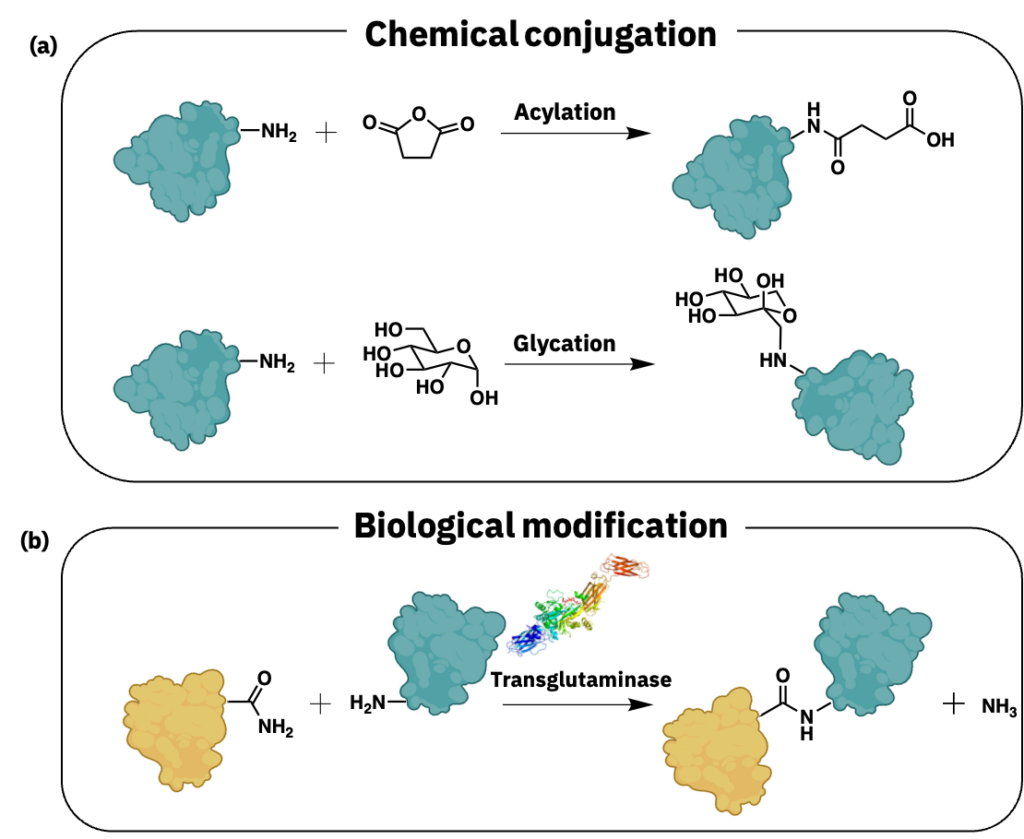
Source: Generic protein blobs produced by BioRender.
Chemical conjugation of proteins with small molecules or macromolecules can significantly alter protein sequence, structure, and functionality. Amino acid groups with reactive moieties, such as a free amine (Figure 3a), hydroxyl, or thiol—for example, lysine, tyrosine, and cysteine, respectively—serve as conjugate sites on proteins. Conjugation increases protein molecular weight and modifies solubility, thermostability, emulsification, and other properties. Chemical conjugation can leave residues and may require further purification, drying, and rehydration of the protein ingredient before use in the formulation.
Glycation via the Maillard reaction occurs naturally during cooking between water, amino acid amino groups, and reducing-sugar carbonyl groups. Its application has also improved protein stability and functionality (Zhang et al. 2018). Adding hydrophilic carbohydrates typically improves water solubility and modifies emulsification and foaming properties. For example, gum arabic conjugation to pea protein hydrolysate improved protein stability and emulsification while reducing beany flavors (Zha et al. 2019). A conjugate of maltodextrin and pea protein isolate in electrospun fibers improved solubility and emulsification (Kutzli et al. 2020a & Kutzli et al. 2020b). Carbohydrate-protein conjugation does not require chemicals other than water, making it a residue-free reaction. But the process conventionally applies prolonged dry heating, hindering scalability and commercial viability (Doost et al. 2019). Other methods to make these types of conjugates should be explored to improve their commercialization potential.
Acylation usually occurs via succinylation, acetylation, or maleylation. Succinylation of pea proteins with various succinic anhydride derivatives significantly modified protein secondary structure and enhanced solubility, foaming capacity and stability, emulsion stability, and water-holding capacity (Shah et al. 2019). Acetylated and succinylated oat protein isolates have been evaluated and generally demonstrated more flexible structures with lowered surface hydrophobicity (Zhao et al. 2017). Succinylation and glycation with dextran of rapeseed protein isolate increased surface hydrophobicity (Wang et al. 2018). Maleylation tested on rapeseed protein isolate increased the protein whiteness and dissociation of proteins, as well as the surface hydrophobicity, foaming capacity, and emulsion stability (Purkayastha et al. 2016).
Lastly, phosphorylation introduces phosphate groups to the amino acid sequence and can be applied via chemical synthesis. Microwave-assisted phosphorylation has been explored for mung bean proteins with tripolyphosphate (Hadidi et al. 2021). The phosphate groups increased the hydrogen bonds, thus increasing water binding. They also added electrostatic repulsion to the protein bodies, thus improving protein dispersibility.
Biological treatments
Many protein modifications are observed in native biological processes aided by enzymes. Such biological processes can be emulated in industrial settings.
Generally performed under mild conditions with nontoxic reactants and byproducts, biological treatments are attractive alternatives to physical and chemical modifications. Many enzymatic reactions are also more specific and faster than many physical and chemical interactions. These attributes help prevent unintended consequences, such as modifying off-target structural properties. Additionally, biological treatments satisfy consumers seeking clean label products. Biological treatments combined with chemical and physical methods can further enhance protein processing (Zhang et al. 2012). This section distinguishes enzymatic treatments from fermentation treatments. The former implies the addition of exogenous enzymes, and the latter relies on enzymes produced in situ by a live microorganism.
Enzymatic modifications of plant proteins include hydrolysis, glycation, crosslinking, and catalyzing the degradation of impurities. Hydrolysis occurs with proteases that break peptide bonds, lowering protein molecular weight and increasing surface ionizable groups and surface hydrophobicity. Alcalase is commonly used for enzymatic hydrolysis to alter protein functionality. For example, alcalase-modified potato protein isolate improved solubility and foaming capacity but lowered foaming stability (Akbari et al. 2019). Additionally, alcalase treatment of chickpea protein isolate improved protein recovery, solubility, and emulsification activity and stability (Ghribi et al. 2015).
Other proteases have also been explored. Oat protein isolate partially hydrolyzed with alcalase, Flavourzyme, pepsin, or trypsin demonstrated significantly improved gel strength with Flavourzyme and trypsin compared with the alcalase- or pepsin-catalyzed oat protein hydrolysates (Nieto-Nieto et al. 2014). In comparing alcalase and flavourzyme applied to peanut protein meal, the meal’s reducing power improved more with alcalase than Flavourzyme or their combination (Hou et al. 2016). Alcalase, neutrase, and trypsin have been applied for enzymatic hydrolysis of rice protein, and trypsin-catalyzed rice protein hydrolysates demonstrated the best emulsion stability (Pan et al. 2019). Thus, protease choice for enzymatic hydrolysis should be optimized for the plant protein source and functional properties of interest.
Enzymes can expedite carbohydrate-protein conjugation. Zhang et al. 2021 formed glycosylated soy protein isolates using transglutaminase with glucosamine. The resulting soy protein isolate-glucosamine conjugate was more flexible, increased surface hydrophobicity, enhanced emulsification capability, and reduced surface tension. Transglutaminase helps form carbohydrate-protein conjugates by catalyzing linkages between amine-containing carbohydrates and γ-carboxamide groups of glutamine-residue side chains.
Transglutaminase can also crosslink proteins (Figure 3b). This interaction occurs between glutamine and the ε-amino groups of lysine residues. This reaction can compete with carbohydrate-protein conjugation, so optimizing reaction conditions is essential (e.g., if glycation is desired, the small molecule amine should be added in excess to prevent protein interactions). Zhang et al. 2018 evaluated the enzymatic crosslinking of black soybean protein isolates and found that 11S globulins were better substrates for transglutaminase than 7S globulins. The authors hypothesized this is because enzymatic interactions rely on enzyme-substrate interactions, so the accessibility of glutamine and lysine residues determines the efficiency of the reaction. In another example, the pea protein fractions globulin and albumin demonstrated different gelation capabilities via enzymatic treatment (Djoullah et al. 2018). Albumin fractions favored aggregate formation over conjugation, which the authors hypothesized was due to the dominant formation of intramolecular bonds. On the other hand, globulin fractions could form gels in native or denatured states—the denatured globulin proteins formed the best gels, likely due to better accessibility of glutamine and lysine residues.
Other enzymes can also induce crosslinking and may be better suited for certain protein characteristics. Oat proteins can crosslink with either transglutaminase or tyrosinase (though tyrosinase has proved less efficient), while faba bean proteins can crosslink only with transglutaminase (Nivala et al. 2017). Phenolic tyrosine residues serve as the substrate for tyrosinase, but some impurities could inhibit the enzyme’s activity.
Enzymatic deamidation is another useful transformation for plant proteins that converts amide groups in glutamine and asparagine groups to carboxyl groups (forming glutamic acid and aspartic acid, respectively). With increased negatively charged carboxyl groups, the resulting proteins have modified properties. For example, glutaminase applied to pea protein isolate resulted in more flexible, extended proteins with smaller average particle sizes (Fang et al. 2020). Compared with untreated protein, pea protein isolates subjected to deamidation have improved solubility, dispersibility, and suspensibility with reduced off-flavors and grittiness.
Enzymes are also commonly used to remove impurities in raw ingredients. Phytase is commonly used to hydrolyze phytic acid, an antinutrient prevalent in many plant protein sources. Kaleda et al. 2020 applied a phytase treatment to an oat and pea protein composite, decreasing phytic acid levels by 32 percent. Another biological treatment, fermentation, made subsequent protein texturization more difficult and enhanced some bitter flavors. GFI grantee Mari-Liis Tammik gave a seminar explaining these findings in depth. While fermentation did not greatly enhance the oat-pea protein mixture, in many cases fermentation has improved attributes and been applied commercially.
The OZO brand, leveraging a partnership between Planterra and MycoTechnology, uses pea and rice protein fermented by shiitake mycelia to improve taste and texture. GFI grantee BZ Goldberg and The Mediterranean Food Lab also apply traditional fermentation to plant ingredients to produce meaty flavor bases for plant-based meat. Studies have demonstrated that lactic acid bacteria effectively reduce antinutrient and off-flavor content in plant proteins. Additionally, these bacteria can produce exopolysaccharides that act as thickeners in the protein solution. Xu et al. 2017 tested the fermentation of faba bean flour with lactic acid bacteria and found that exopolysaccharide formation was promoted by sucrose addition and use of Leuconostoc spp. (instead of Weissella spp.). As a result, this bacterium promoted stable gel formation of faba bean flour.
One study combined dry fractionation and fermentation for chickpea protein flour, which lowered the flour’s antinutrient content and increased its water-holding capacity (Xing et al. 2020). Other studies have demonstrated that fermentation can lower antinutrient content in soybean meal (Yasar et al. 2020) and improve the aroma profile of lupin protein flour (Kaczmarska et al. 2018). Biomass and precision fermentation processes are also useful for plant-based hybrid products and are discussed in more detail on GFI’s science of fermentation web page.
Opportunities to improve enzymatic and fermentation methods for protein functionalization abound, as highlighted in GFI’s solution concept note on biological processing. Existing enzymes and microbial strains may require selection, adaptation, screening, or engineering to enhance protein enrichment and modification or to optimize their function for specific target substrates. Additionally, standardized combinations of biological treatments with physical and chemical methods should be explored to identify broader insights and reduce the time and resources necessary for protein processing optimization. Because protein properties are extremely dependent on processing methods, bench-scale optimization alongside techno-economic models for industrial-scale processes can better inform development, as described in GFI’s solution on open-access techno-economic assessments.
